Search results
Search for "hybrid compounds" in Full Text gives 12 result(s) in Beilstein Journal of Organic Chemistry.
Recent developments in the engineered biosynthesis of fungal meroterpenoids
- Zhiyang Quan and
- Takayoshi Awakawa
Beilstein J. Org. Chem. 2024, 20, 578–588, doi:10.3762/bjoc.20.50
- Zhiyang Quan Takayoshi Awakawa RIKEN Center for Sustainable Resource Science, Wako, Saitama 351-0198, Japan 10.3762/bjoc.20.50 Abstract Meroterpenoids are hybrid compounds that are partially derived from terpenoids. This group of natural products displays large structural diversity, and many

Graphical Abstract

Figure 1: Examples of bioactive fungal meroterpenoids.

Figure 2: The diversity of DMOA-derived meroterpenoid biosyntheses.

Figure 3: The combinatorial biosynthesis of diterpene pyrone meroterpenoids. The production of subglutinol A ...

Figure 4: The biosynthetic reaction from the common intermediate 21 to ascochlorin (22) and ascofuranone (23)...

Figure 5: The multistep oxidations catalyzed by AusE and PrhA from the common intermediate 24.

Figure 6: Reactions of SptF with native substrates 31 and 32.

Figure 7: A) Reactions of SptF with unnatural substrates. B) Reactions of SptF variants with 31.

Figure 8: The reaction of the αKG enzyme AndA and its variants generated via saturated mutagenesis.

Figure 9: The synthetic biological production of daurichromenic acid and its halogenated derivative.
Synthesis of imidazo[4,5-e][1,3]thiazino[2,3-c][1,2,4]triazines via a base-induced rearrangement of functionalized imidazo[4,5-e]thiazolo[2,3-c][1,2,4]triazines
- Dmitry B. Vinogradov,
- Alexei N. Izmest’ev,
- Angelina N. Kravchenko,
- Yuri A. Strelenko and
- Galina A. Gazieva
Beilstein J. Org. Chem. 2023, 19, 1047–1054, doi:10.3762/bjoc.19.80
- closely related hybrid compounds including fragments of 1,3-thiazine and imidazo-1,2,4-triazine is still highly relevant. Earlier we have demonstrated that imidazo[4,5-e]thiazolo[3,2-b]-1,2,4-triazines and their derivatives functionalized at position 6 are capable of undergoing skeletal rearrangements and

Graphical Abstract

Figure 1: Examples of natural and synthetic bioactive 1,3-thiazine and imidazothiazolotriazine derivatives wi...

Scheme 1: Base-induced transformations and rearrangements of functionalized imidazo[4,5-e]thiazolo[3,2-b]-1,2...

Scheme 2: Alkaline hydrolysis of esters 1a,b. aDetermined by 1H NMR spectroscopy; bisolated yields.

Scheme 3: Synthesis of potassium imidazo[4,5-e][1,3]thiazino[2,3-c][1,2,4]triazine-7-carboxylates.

Scheme 4: Plausible rearrangement mechanism of imidazo[4,5-e]thiazolo[2,3-c][1,2,4]triazine 1d into imidazo[4...

Figure 2: 1H NMR spectra of the starting compound 1d (a) and the reaction mixture after 1.5 (b) and 4 (c) hou...

Scheme 5: Synthetic approaches to imidazo[4,5-e][1,3]thiazino[2,3-c][1,2,4]triazines 3a–d,j.

Scheme 6: Synthesis of imidazo[4,5-e][1,3]thiazino[2,3-c][1,2,4]triazine-7-carboxylic acids 5a–j.

Scheme 7: Synthesis of imidazo[4,5-e][1,3]thiazino[2,3-c][1,2,4]triazine-7-carboxylic acids 5k,m.

Scheme 8: Plausible path for the formation of products 9.

Figure 3: 1H NMR spectra of compounds 4a and 5a in DMSO-d6 in the region of 4.3–9.0 ppm.

Figure 4: 13C NMR GATED spectra of compounds 4a and 5a in DMSO-d6 in the region of 156.0–168.0 ppm.

Figure 5: General view of 5a in the crystal in thermal ellipsoid representation (p = 80%).
Cholyl 1,3,4-oxadiazole hybrid compounds: design, synthesis and antimicrobial assessment
- Anas J. Rasras,
- Mohamed El-Naggar,
- Nesreen A. Safwat and
- Raed A. Al-Qawasmeh
Beilstein J. Org. Chem. 2022, 18, 631–638, doi:10.3762/bjoc.18.63

Graphical Abstract

Figure 1: Biologically active cholic acid hybridized with different heterocyclic scaffolds.

Scheme 1: Synthesis of cholyl 1,3,4-oxadiazole-2-thiol 2.

Scheme 2: Synthesis of cholyl 2-(propargylthio)-1,3,4-oxadiazole 3.

Scheme 3: Synthesis of target compounds 4a–v.

Figure 2: Structures of target compounds 4a–v.
The use of isoxazoline and isoxazole scaffolding in the design of novel thiourea and amide liquid-crystalline compounds
- Itamar L. Gonçalves,
- Rafaela R. da Rosa,
- Vera L. Eifler-Lima and
- Aloir A. Merlo
Beilstein J. Org. Chem. 2020, 16, 175–184, doi:10.3762/bjoc.16.20
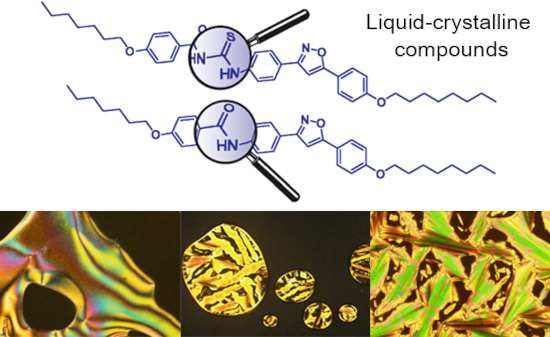
- previously published [19][21][22][23][24]. The synthetic route used for the synthesis of the hybrid compounds, N-acyl-N'-isoxazolinylthioureas 17a–c and N-acyl-N'- isoxazolylthioureas 18a–c is outlined in Scheme 2. The synthesis of thioureas 17a–c and 18a–c involved the activation of 4-heptyloxybenzoic acid

Graphical Abstract

Scheme 1: Amines 3, 4, 8, 9, 12 and 13 installed on 5-membered isoxazoline and isoxazole rings.

Scheme 2: Synthesis of acylisoxazolinylthioureas 17a–c and acylisoxazolylthioureas 18a–c. (i) SOCl2, reflux, ...

Scheme 3: Synthesis of amides. Part A: (i) SOCl2, reflux; (ii) KOCN, acetone, reflux; (iii) amines 3, 4, 8 an...

Figure 1: Optical textures observed on POM for thioureas 17a (a), 17b (b), 18a (c), 18b (d) and 18c (e,f). Al...

Figure 2: Optical textures observed on POM of amide 19. (a) Fan-shaped focal conic texture of the SmA mesopha...

Figure 3: DSC curves for the thiourea 17a (A), amide 19 (B) and 20 (C) upon the first heating and cooling cur...

Figure 4: TGA analysis for thiourea 18c; and amides 20 and 22.
Combining the Ugi-azide multicomponent reaction and rhodium(III)-catalyzed annulation for the synthesis of tetrazole-isoquinolone/pyridone hybrids
- Gerardo M. Ojeda,
- Prabhat Ranjan,
- Pavel Fedoseev,
- Lisandra Amable,
- Upendra K. Sharma,
- Daniel G. Rivera and
- Erik V. Van der Eycken
Beilstein J. Org. Chem. 2019, 15, 2447–2457, doi:10.3762/bjoc.15.237
- could form isoquinolones and pyridones linked to a tetrazole ring, since such class of hybrid compounds is not described in the literature. Several protocols based on C–H activation or metal-catalyzed cyclizations are known to generate the isoquinolone and pyridone moieties and further assist their post
- explored with substrates bearing acyl groups other than benzoyl (Scheme 5). Compounds incorporating acrylamidomethyltetrazole fragments reacted with diphenylacetylene to form the tetrazole/pyridone hybrids 5a–c in good yields. In addition, very complex hybrid compounds based on heterocyclic, fused aromatic
- reactions (MCRs) with the large synthetic scope of metal-catalyzed cyclization protocols [49][50][51][52][53][54]. In this sense, we envisioned that the application of modern Ru(II) or Rh(III)-catalyzed annulations on Ugi-azide-4CR-derived scaffolds could be a promising strategy to generate novel hybrid

Graphical Abstract

Figure 1: Bioactive molecules containing a tetrazole, pyridone or isoquinolone ring.

Scheme 1: Approaches for the synthesis of tetrazoles and isoquinolones and their interplay as designed in thi...

Scheme 2: Scope of the Ugi-azide-4CR/deprotection/acylation sequence. Ugi-azide-4CR conducted at the 2.0 mmol...

Scheme 3: Influence of substituents R and R2 on the reaction outcome. For compounds 4k–m the overall yield in...

Scheme 4: Influence of the alkyne and R1 substituent on the reaction outcome.

Scheme 5: Scope of acrylic, heterocyclic and ring-fused N-acylaminomethyl tetrazole substrates.

Scheme 6: Proposed reaction mechanism using substrates 1a and 3a.
Steroid diversification by multicomponent reactions
- Leslie Reguera,
- Cecilia I. Attorresi,
- Javier A. Ramírez and
- Daniel G. Rivera
Beilstein J. Org. Chem. 2019, 15, 1236–1256, doi:10.3762/bjoc.15.121
- the preparation of steroidal derivatives with a pyrimidine moiety fused to ring D. This heterocycle moiety appears in different biologically active steroids fused to ring D, and previous non-MCR methods had been reported for the creation of libraries of such hybrid compounds [40]. The Biginelli

Graphical Abstract

Figure 1: Structures of natural steroids of A) animal and B) plant origin.

Scheme 1: Synthesis of a steroidal β-lactam by Ugi reaction of a cholanic aldehyde [14].

Scheme 2: Synthetic route to steroidal 2,5-diketopiperazines based on a diastereoselective Ugi-4CR with an an...

Scheme 3: Multicomponent synthesis of a heterocycle–steroid hybrid using a ketosteroid as carbonyl component [18]....

Scheme 4: Synthesis of peptidomimetic–steroid hybrids using the Ugi-4CR with spirostanic amines and carboxyli...

Scheme 5: Synthesis of azasteroids using the Ugi-4CR with androstanic and pregnanic carboxylic acids [22].

Figure 2: Ugi-4CR-derived library of androstanic azasteroids with diverse substitution patterns at the phenyl...

Scheme 6: Synthesis of 4-azacholestanes by an intramolecular Ugi-4C-3R [26].

Scheme 7: Synthesis of amino acid–steroid hybrid by multiple Ugi-4CR using steroidal isocyanides [29].

Scheme 8: Synthesis of ecdysteroid derivatives by Ugi-4CR using a steroidal isocyanide [30].

Scheme 9: Stereoselective multicomponent synthesis of a steroid–tetrahydropyridine hybrid using a chiral bifu...

Scheme 10: Pd(II)-catalyzed three-component reaction with an alkynyl seco-cholestane [34].

Scheme 11: Multicomponent synthesis of steroid–thiazole hybrids from a steroidal ketone [36].

Scheme 12: Synthesis of cholanic pseudo-peptide derivatives by novel MCRs based on the reactivity of ynamide [37,38].

Scheme 13: Synthesis of steroid-fused pyrimidines and pyrimidones using the Biginelli-3CR [39,42,43].

Scheme 14: Synthesis of steroidal pyridopyrimidines by a reaction sequence comprising a 4CR followed by a post...

Scheme 15: Synthesis of steroid-fused pyrimidines by MCR of 2-hydroxymethylene-3-ketosteroids [46].

Scheme 16: Synthesis of steroid-fused naphthoquinolines by the Kozlov–Wang MCR using ketosteroids [50,51].

Scheme 17: Conjugation of steroids to carbohydrates and peptides by the Ugi-4CR [62,63].

Scheme 18: Solid-phase multicomponent conjugation of peptides to steroids by the Ugi-4CR [64].

Scheme 19: Solid-phase multicomponent conjugation of peptides to steroids by the Petasis-3CR [68].

Scheme 20: Synthesis of steroidal macrobicycles (cages) by multiple multicomponent macrocyclizations based on ...

Scheme 21: One-pot synthesis of steroidal cages by double Ugi-4CR-based macrocyclizations [76].
Synthesis of 3-aminocoumarin-N-benzylpyridinium conjugates with nanomolar inhibitory activity against acetylcholinesterase
- Nisachon Khunnawutmanotham,
- Cherdchai Laongthipparos,
- Patchreenart Saparpakorn,
- Nitirat Chimnoi and
- Supanna Techasakul
Beilstein J. Org. Chem. 2018, 14, 2545–2552, doi:10.3762/bjoc.14.231
- . Hybrid compounds such as huperzine A–tacrine hybrids [5], donepezil–tacrine hybrid related derivatives [6][7] and tacrine–indole hybrids [8] with dual-binding site properties exhibit potent AChE inhibition activities. In addition, coumarin compounds linked with various side chain moieties [9][10][11] as

Graphical Abstract

Figure 1: Design of the target compounds.

Scheme 1: Synthesis of 3-aminocoumarin-N-benzylpyridinium salts.

Figure 2: Docked conformations of donepezil (ball-and-stick model; pink), compounds 9a, 9b, 9e, 9h, and 9i (s...

Figure 3: Binding interactions in the rhAChE binding pocket with (a) 4a, (b) 9a, (c) 9b, (d) 9e, (e) 9h, (f) ...
Synthesis and spectroscopic properties of β-meso directly linked porphyrin–corrole hybrid compounds
- Baris Temelli and
- Hilal Kalkan
Beilstein J. Org. Chem. 2018, 14, 187–193, doi:10.3762/bjoc.14.13
- -5,10,15,20-tetraphenylporphyrins with meso-substituted dipyrromethanes. Hybrid compounds have been characterized by 1H NMR, 13C NMR, 2D NMR, UV–vis absorption and fluorescence spectroscopy. Keywords: corrole; hybrid compounds; indium(III) chloride; porphyrin; porphyrinoids; Introduction Porphyrins and
- intramolecular energy transfer between macrocycles [38]. As a part of our ongoing research on porphyrin–corrole conjugates, herein we describe a convenient synthesis of a series of novel directly linked porphyrin–corrole hybrid compounds. For this purpose, acid-catalyzed reactions of dipyrromethanes and
- with aldehydes [42]. When electron-withdrawing 4-nitrophenyl and pentafluorophenyl substituents were used on the dipyrromethane, hybrid compounds 4c and 4e were isolated in 16% and 20% yields, respectively. The synthesis of 4e with InCl3 afforded a very low yield, thus an equimolar amount of AlCl3 was

Graphical Abstract

Scheme 1: Overview of the synthesis of directly linked porphyrin–corrole hybrid compounds.

Scheme 2: Synthesis of β-meso directly linked porphyrin–corrole hybrid compounds.

Scheme 3: Synthesis of porphyrin–corrole hybrid derivatives. *100 mol % of AlCl3 was used as a catalyst.

Figure 1: 1H NMR spectrum of 4a in CDCl3.
Dicarboxylic esters: Useful tools for the biocatalyzed synthesis of hybrid compounds and polymers
- Ivan Bassanini,
- Karl Hult and
- Sergio Riva
Beilstein J. Org. Chem. 2015, 11, 1583–1595, doi:10.3762/bjoc.11.174
- different bioactive molecules with suitable dicarboxylic acids to prepare hybrid compounds has been receiving more and more attention. The interest is due to the fact that these new substances might show additive activities [36], having improved properties or efficacies compared to the combined use of the
- once again the well-known efficiency, selectivity and versatility of CAL-B (Novozyme 435) [16]. As in the previous examples, the mixed esters from the first esterification step can be used as acylating agents in the second esterification step. Scheme 3 shows the synthesis of the hybrid compounds 17 and

Graphical Abstract

Scheme 1: Activated derivatives of dicarboxylic acids.

Figure 1: Example of natural compounds selectively acylated with dicarboxylic esters.

Figure 2: C6-dicarboxylic acid diesters derivatives of NAG-thiazoline.

Figure 3: Sylibin dimers obtained by CAL-B catalyzed trans-acylation reactions.

Scheme 2: Biocatalyzed synthesis of paclitaxel derivatives.

Figure 4: 5-Fluorouridine derivatives obtained by CAL-B catalysis.

Scheme 3: Biocatalyzed synthesis of hybrid diesters 17 and 18.

Scheme 4: Hybrid derivatives of sylibin.

Figure 5: Bolaamphiphilic molecules containing (L)- and/or (D)-isoascorbic acid moieties.

Figure 6: Doxorubicin (29) trapped in a polyester made of glycolate, sebacate and 1,4-butandiol units.

Figure 7: Polyesters containing functionalized pentofuranose derivatives.

Figure 8: Polyesters containing disulfide moieties.

Figure 9: Polyesters containing epoxy moieties.

Figure 10: Biocatalyzed synthesis of polyesters containing glycerol.

Figure 11: Iataconic (34) and malic (35) acid.

Figure 12: Oxidized poly(hexanediol-2-mercaptosuccinate) polymer.

Figure 13: C-5-substituted isophthalates.

Figure 14: Curcumin-based polyesters.

Figure 15: Silylated polyesters.

Figure 16: Polyesters containing reactive ether moieties.

Figure 17: Polyesters obtained by CAL-B-catalyzed condensation of dicarboxylic esters and N-substituted dietha...

Figure 18: Polyesters comprising mexiletine (38) moieties.

Figure 19: Poly(amide-co-ester)s comprising a terminal hydroxy moiety.

Figure 20: Polymer comprising α-oxydiacid moieties.

Figure 21: Telechelics with methacrylate ends.

Figure 22: Telechelics with allyl-ether ends.

Figure 23: Telechelics with ends functionalized as epoxides.
Multicomponent reactions in nucleoside chemistry
- Mariola Koszytkowska-Stawińska and
- Włodzimierz Buchowicz
Beilstein J. Org. Chem. 2014, 10, 1706–1732, doi:10.3762/bjoc.10.179
- moderate yields. Antiparasitic activities of the hybrid compounds 15 were evaluated; some of them showed moderate activities against trypomastigote forms of Trypanosoma brucei brucei GVR 35 (e.g., IC50 = 25 µM for Ar = C6H4-Cl-4). The Mannich reaction was also used to construct nucleoside scaffolds from
- . Activity of hybrid compounds 28 and 29 against VZV and CMV viruses, as well as against Leishmania donovani promastigotes, was evaluated. Unfortunately, none of them showed any activity up to 250 μM. 3. The Ugi reaction The Ugi reaction allows for a facile synthesis of a bisamide from a ketone (or an

Graphical Abstract

Figure 1: Selected chemical modifications of natural ribose or 2'-deoxyribose nucleosides leading to the deve...

Scheme 1: (a) Classical Mannich reaction; (b) general structures of selected hydrogen active components and s...

Scheme 2: Reagents and reaction conditions: i. H2O or H2O/EtOH, 60–100 °C, 7 h–10 d; ii. H2, Pd/C or PtO2; ii...

Scheme 3: Reagents and reaction conditions: i. H2O, 90 °C, overnight.

Scheme 4: Reagents and reaction conditions: i. AcOH, H2O, 60 °C, 12 h-5 d; ii. AcOH, H2O, 60 °C, 8 h.

Scheme 5: Reagents and reaction conditions: i. CuBr, THF, reflux, 0.5 h; ii. n-Bu4NF·3H2O, THF, rt, 2 h.

Scheme 6: Reagents and reaction conditions: i. [bmim][PF6], 80 °C, 5–8 h.

Scheme 7: Reagents and reaction conditions: i. EtOH, reflux, 24 h.

Scheme 8: Reagents and reaction conditions: i. NaOAc, H2O, 95 °C, 1–16 h; ii. NaOAc, H2O, 95 °C, 1 h.

Scheme 9: Reagents and reaction conditions: i. a. 37% aq HCl, MeOH; b. NaOAc, 1,4-dioxane, H2O, 100 °C, overn...

Scheme 10: Reagents and reaction conditions: i. DMAP, DCC, MeOH, rt, 1 h.

Scheme 11: The Kabachnik–Fields reaction.

Scheme 12: Reagents and reaction conditions: i. 60 °C, 3 h; ii. 80 °C, 2 h.

Scheme 13: The four-component Ugi reaction.

Scheme 14: Reagents and reaction conditions: i. MeOH, rt, 2–3 d, yields not given.

Scheme 15: Reagents and reaction conditions: i. MeOH/CH2Cl2 (1:1), rt, 24 h, yield not given; ii. 6 N aq HCl, ...

Scheme 16: Reagents and reaction conditions: i. MeOH/H2O, rt, 26 h; ii. aq AcOH, reflux, 50%; iii. reversed ph...

Scheme 17: Reagents and reaction conditions: i. MeOH, rt, 24 h; ii. HCl, MeOH, 0 °C to rt, 6 h, then H2O, rt, ...

Scheme 18: Reagents and reaction conditions: i. DMF/Py/MeOH (1:1:1), rt, 48 h; ii. 10% HCl/MeOH, rt, 30 min.

Scheme 19: Reagents and reaction conditions (R = CH3 or H): i. CH2Cl2/MeOH (2:1), 35–40 °C, 2 d; ii. HF/pyridi...

Scheme 20: Reagents and reaction conditions: i. MeOH, 76%; ii. 80% aq TFA, 100%.

Scheme 21: Reagents and reaction conditions: i. EtOH, rt, 72 h; ii. Zn, aq NaH2PO4, THF, rt, 1 week; then 80% ...

Scheme 22: Reagents and reaction conditions: i. EtOH, rt, 48 h, then silica gel chromatography, 33% for 57 (30...

Scheme 23: Reagents and reaction conditions: i. [bmim]BF4, 80 °C, 4 h; ii. [bmim]BF4, 80 °C, 3 h; iii. [bmim]BF...

Scheme 24: Reagents and reaction conditions: i. [bmim]BF4, 80 °C.

Scheme 25: Reagents and reaction conditions: i. H3PW12O40 (2 mol %), EtOH, 50 °C, 2–15 h; ii. H3PW12O40 (2 mol...

Scheme 26: General scheme of the Biginelli reaction.

Scheme 27: Reagents and reaction conditions: i. EtOH, reflux.

Scheme 28: Reagents and reaction conditions: i. Bu4N+HSO4−, diethylene glycol, 120 °C, 1.5–3 h.

Scheme 29: Reagents and reaction conditions: i. BF3·Et2O, CuCl, AcOH, THF, 65 °C, 24 h; ii. Yb(OTf)3, THF, ref...

Scheme 30: Reagents and reaction conditions: TCT (10 mol %), rt: i. 100 min; ii. 150 min; iii. 140 min.

Scheme 31: Reagents and reaction conditions: i. EtOH, microwave irradiation (300 W), 10 min; ii. EtOH, 75 °C, ...

Scheme 32: The Hantzsch reaction.

Scheme 33: Reagents and reaction conditions: TCT (10 mol %), rt, 80–150 min.

Scheme 34: Reagents and reaction conditions: i. Yb(OTf)3, THF, 90 °C, 12 h; ii. 4 Å molecular sieves, EtOH, 90...

Scheme 35: Reagents and reaction conditions: i. MeOH, 50 °C, 48 h.

Scheme 36: Reagents and reaction conditions: i. MeOH, 25 °C, 5 d.

Scheme 37: Bu4N+HSO4−, diethylene glycol, 80 °C, 1–2 h.

Scheme 38: The three-component carbopalladation of dienes on the example of buta-1,3-diene.

Scheme 39: Reagents and reaction conditions: i. 5 mol % Pd(dba)2, Bu4NCl, ZnCl2, acetonitrile or DMSO, 80 °C o...

Scheme 40: Reagents and reaction conditions: i. 2.5 mol % Pd2(dba)3, tris(2-furyl)phosphine, K2CO3, MeCN or DM...

Scheme 41: Reagents and reaction conditions: i. 2.5 mol % Pd2(dba)3, tris(2-furyl)phosphine, K2CO3, MeCN or DM...

Scheme 42: The three-component Bucherer–Bergs reaction.

Scheme 43: Reagents and reaction conditions: i. MeOH, H2O, 70 °C, 4.5 h; ii. (1) H2, 5% Pd/C, MeOH, 55 °C, 5 h...

Scheme 44: Reagents and reaction conditions: i. pyridine, MgSO4, 100 °C, 28 h, N2; ii. DMF, 70–90 °C, 22–30 h,...

Scheme 45: Reagents and reaction conditions: i. Montmorillonite K-10 clay, microwave irradiation (600 W), 6–10...

Scheme 46: Reagents and reaction conditions: i. Montmorillonite K-10 clay, microwave irradiation (560 W), 6–10...

Scheme 47: Reagents and reaction conditions: i. CeCl3·7H2O (20 mol %), NaI (20 mol %), microwave irradiation (...

Scheme 48: Reagents and reaction conditions: i. PhI(OAc)2 (3 mol %), microwave irradiation (45 °C), 6–9 min.

Scheme 49: Reagents and reaction conditions: i. 117, ethyl pyruvate, TiCl4, dichloromethane, −78 °C, 1 h; then ...
Synthesis of five- and six-membered cyclic organic peroxides: Key transformations into peroxide ring-retaining products
- Alexander O. Terent'ev,
- Dmitry A. Borisov,
- Vera A. Vil’ and
- Valery M. Dembitsky
Beilstein J. Org. Chem. 2014, 10, 34–114, doi:10.3762/bjoc.10.6

Graphical Abstract

Figure 1: Five and six-membered cyclic peroxides.

Figure 2: Artemisinin and semi-synthetic derivatives.

Scheme 1: Synthesis of 3-hydroxy-1,2-dioxolanes 3a–c.

Scheme 2: Synthesis of dioxolane 6.

Scheme 3: Photooxygenation of oxazolidines 7a–d with formation of spiro-fused oxazolidine-containing dioxolan...

Scheme 4: Oxidation of cyclopropanes 10a–e and 11a–e with preparation of 1,2-dioxolanes 12a–e.

Scheme 5: VO(acac)2-catalyzed oxidation of silylated bicycloalkanols 13a–c.

Scheme 6: Mn(II)-catalyzed oxidation of cyclopropanols 15a–g.

Scheme 7: Oxidation of aminocyclopropanes 20a–c.

Scheme 8: Synthesis of aminodioxolanes 24.

Figure 3: Trifluoromethyl-containing dioxolane 25.

Scheme 9: Synthesis of 1,2-dioxolanes 27a–e by the oxidation of cyclopropanes 26a–e.

Scheme 10: Photoinduced oxidation of methylenecyclopropanes 28.

Scheme 11: Irradiation-mediated oxidation.

Scheme 12: Application of diazene 34 for dioxolane synthesis.

Scheme 13: Mn(OAc)3-catalyzed cooxidation of arylacetylenes 37a–h and acetylacetone with atmospheric oxygen.

Scheme 14: Peroxidation of (2-vinylcyclopropyl)benzene (40).

Scheme 15: Peroxidation of 1,4-dienes 43a,b.

Scheme 16: Peroxidation of 1,5-dienes 46.

Scheme 17: Peroxidation of oxetanes 53a,b.

Scheme 18: Peroxidation of 1,6-diene 56.

Scheme 19: Synthesis of 3-alkoxy-1,2-dioxolanes 62a,b.

Scheme 20: Synthesis of spiro-bis(1,2-dioxolane) 66.

Scheme 21: Synthesis of dispiro-1,2-dioxolanes 68, 70, 71.

Scheme 22: Synthesis of spirohydroperoxydioxolanes 75a,b.

Scheme 23: Synthesis of spirohydroperoxydioxolane 77 and dihydroperoxydioxolane 79.

Scheme 24: Ozonolysis of azepino[4,5-b]indole 80.

Scheme 25: SnCl4-mediated fragmentation of ozonides 84a–l in the presence of allyltrimethylsilane.

Scheme 26: SnCl4-mediated fragmentation of bicyclic ozonide 84m in the presence of allyltrimethylsilane.

Scheme 27: MCl4-mediated fragmentation of alkoxyhydroperoxides 96 in the presence of allyltrimethylsilane.

Scheme 28: SnCl4-catalyzed reaction of monotriethylsilylperoxyacetal 108 with alkene 109.

Scheme 29: SnCl4-catalyzed reaction of triethylsilylperoxyacetals 111 with alkenes.

Scheme 30: Desilylation of tert-butyldimethylsilylperoxy ketones 131a,b followed by cyclization.

Scheme 31: Deprotection of peroxide 133 followed by cyclization.

Scheme 32: Asymmetric peroxidation of methyl vinyl ketones 137a–e.

Scheme 33: Et2NH-catalyzed intramolecular cyclization.

Scheme 34: Synthesis of oxodioxolanes 143a–j.

Scheme 35: Haloperoxidation accompanied by intramolecular ring closure.

Scheme 36: Oxidation of triterpenes 149a–d with Na2Cr2O7/N-hydroxysuccinimide.

Scheme 37: Curtius and Wolff rearrangements to form 1,2-dioxolane ring-retaining products.

Scheme 38: Oxidative desilylation of peroxide 124.

Scheme 39: Synthesis of dioxolane 158, a compound containing the aminoquinoline antimalarial pharmacophore.

Scheme 40: Diastereomers of plakinic acid A, 162a and 162b.

Scheme 41: Ozonolysis of alkenes.

Scheme 42: Cross-ozonolysis of alkenes 166 with carbonyl compounds.

Scheme 43: Ozonolysis of the bicyclic cyclohexenone 168.

Scheme 44: Cross-ozonolysis of enol ethers 172a,b with cyclohexanone.

Scheme 45: Griesbaum co-ozonolysis.

Scheme 46: Reactions of aryloxiranes 177a,b with oxygen.

Scheme 47: Intramolecular formation of 1,2,4-trioxolane 180.

Scheme 48: Formation of 1,2,4-trioxolane 180 by the reaction of 1,5-ketoacetal 181 with H2O2.

Scheme 49: 1,2,4-Trioxolane 186 with tetrazole fragment.

Scheme 50: 1,2,4-Trioxolane 188 with a pyridine fragment.

Scheme 51: 1,2,4-Trioxolane 189 with pyrimidine fragment.

Scheme 52: Synthesis of aminoquinoline-containing 1,2,4-trioxalane 191.

Scheme 53: Synthesis of arterolane.

Scheme 54: Oxidation of diarylheptadienes 197a–c with singlet oxygen.

Scheme 55: Synthesis of hexacyclinol peroxide 200.

Scheme 56: Oxidation of enone 201 and enenitrile 203 with singlet oxygen.

Scheme 57: Synthesis of 1,2-dioxanes 207 by oxidative coupling of carbonyl compounds 206 and alkenes 205.

Scheme 58: 1,2-Dioxanes 209 synthesis by co-oxidation of 1,5-dienes 208 and thiols.

Scheme 59: Synthesis of bicyclic 1,2-dioxanes 212 with aryl substituents.

Scheme 60: Isayama–Mukaiyama peroxysilylation of 1,5-dienes 213 followed by desilylation under acidic conditio...

Scheme 61: Synthesis of bicycle 218 with an 1,2-dioxane ring.

Scheme 62: Intramolecular cyclization with an oxirane-ring opening.

Scheme 63: Inramolecular cyclization with the oxetane-ring opening.

Scheme 64: Intramolecular cyclization with the attack on a keto group.

Scheme 65: Peroxidation of the carbonyl group in unsaturated ketones 228 followed by cyclization of hydroperox...

Scheme 66: CsOH and Et2NH-catalyzed cyclization.

Scheme 67: Preparation of peroxyplakoric acid methyl ethers A and D.

Scheme 68: Hg(OAc)2 in 1,2-dioxane synthesis.

Scheme 69: Reaction of 1,4-diketones 242 with hydrogen peroxide.

Scheme 70: Inramolecular cyclization with oxetane-ring opening.

Scheme 71: Inramolecular cyclization with MsO fragment substitution.

Scheme 72: Synthesis of 1,2-dioxane 255a, a structurally similar compound to natural peroxyplakoric acids.

Scheme 73: Synthesis of 1,2-dioxanes based on the intramolecular cyclization of hydroperoxides containing C=C ...

Scheme 74: Use of BCIH in the intramolecular cyclization.

Scheme 75: Palladium-catalyzed cyclization of δ-unsaturated hydroperoxides 271a–e.

Scheme 76: Intramolecular cyclization of unsaturated peroxyacetals 273a–d.

Scheme 77: Allyltrimethylsilane in the synthesis of 1,2-dioxanes 276a–d.

Scheme 78: Intramolecular cyclization using the electrophilic center of the peroxycarbenium ion 279.

Scheme 79: Synthesis of bicyclic 1,2-dioxanes.

Scheme 80: Preparation of 1,2-dioxane 286.

Scheme 81: Di(tert-butyl)peroxalate-initiated radical cyclization of unsaturated hydroperoxide 287.

Scheme 82: Oxidation of 1,4-betaines 291a–d.

Scheme 83: Synthesis of aminoquinoline-containing 1,2-dioxane 294.

Scheme 84: Synthesis of the sulfonyl-containing 1,2-dioxane.

Scheme 85: Synthesis of the amido-containing 1,2-dioxane 301.

Scheme 86: Reaction of singlet oxygen with the 1,3-diene system 302.

Scheme 87: Synthesis of (+)-premnalane А and 8-epi-premnalane A.

Scheme 88: Synthesis of the diazo group containing 1,2-dioxenes 309a–e.

Figure 4: Plakortolide Е.

Scheme 89: Synthesis of 6-epiplakortolide Е.

Scheme 90: Application of Bu3SnH for the preparation of tetrahydrofuran-containing bicyclic peroxides 318a,b.

Scheme 91: Application of Bu3SnH for the preparation of lactone-containing bicyclic peroxides 320a–f.

Scheme 92: Dihydroxylation of the double bond in the 1,2-dioxene ring 321 with OsO4.

Scheme 93: Epoxidation of 1,2-dioxenes 324.

Scheme 94: Cyclopropanation of the double bond in endoperoxides 327.

Scheme 95: Preparation of pyridazine-containing bicyclic endoperoxides 334a–c.

Scheme 96: Synthesis of 1,2,4-trioxanes 337 by the hydroperoxidation of unsaturated alcohols 335 with 1O2 and ...

Scheme 97: Synthesis of sulfur-containing 1,2,4-trioxanes 339.

Scheme 98: BF3·Et2O-catalyzed synthesis of the 1,2,4-trioxanes 342a–g.

Scheme 99: Photooxidation of enol ethers or vinyl sulfides 343.

Scheme 100: Synthesis of tricyclic peroxide 346.

Scheme 101: Reaction of endoperoxides 348a,b derived from cyclohexadienes 347a,b with 1,4-cyclohexanedione.

Scheme 102: [4 + 2]-Cycloaddition of singlet oxygen to 2Н-pyrans 350.

Scheme 103: Synthesis of 1,2,4-trioxanes 354 using peroxysilylation stage.

Scheme 104: Epoxide-ring opening in 355 with H2O2 followed by the condensation of hydroxy hydroperoxides 356 wi...

Scheme 105: Peroxidation of unsaturated ketones 358 with the H2O2/CF3COOH/H2SO4 system.

Scheme 106: Synthesis of 1,2,4-trioxanes 362 through Et2NH-catalyzed intramolecular cyclization.

Scheme 107: Reduction of the double bond in tricyclic peroxides 363.

Scheme 108: Horner–Wadsworth–Emmons reaction in the presence of peroxide group.

Scheme 109: Reduction of ester group by LiBH4 in the presence of 1,2,4-trioxane moiety.

Scheme 110: Reductive amination of keto-containing 1,2,4-trioxane 370.

Scheme 111: Reductive amination of keto-containing 1,2,4-trioxane and a Fe-containing moiety.

Scheme 112: Acid-catalyzed reactions of Н2О2 with ketones and aldehydes 374.

Scheme 113: Cyclocondensation of carbonyl compounds 376a–d using Me3SiOOSiMe3/CF3SO3SiMe3.

Scheme 114: Peroxidation of 4-methylcyclohexanone (378).

Scheme 115: Synthesis of symmetrical tetraoxanes 382a,b from aldehydes 381a,b.

Scheme 116: Synthesis of unsymmetrical tetraoxanes using of MeReO3.

Scheme 117: Synthesis of symmetrical tetraoxanes using of MeReO3.

Scheme 118: Synthesis of symmetrical tetraoxanes using of MeReO3.

Scheme 119: MeReO3 in the synthesis of symmetrical tetraoxanes with the use of aldehydes.

Scheme 120: Preparation of unsymmmetrical 1,2,4,5-tetraoxanes with high antimalarial activity.

Scheme 121: Re2O7-Catalyzed synthesis of tetraoxanes 398.

Scheme 122: H2SO4-Catalyzed synthesis of steroidal tetraoxanes 401.

Scheme 123: HBF4-Catalyzed condensation of bishydroperoxide 402 with 1,4-cyclohexanedione.

Scheme 124: BF3·Et2O-Catalyzed reaction of gem-bishydroperoxides 404 with enol ethers 405 and acetals 406.

Scheme 125: HBF4-Catalyzed cyclocondensation of bishydroperoxide 410 with ketones.

Scheme 126: Synthesis of symmetrical and unsymmetrical tetraoxanes 413 from benzaldehydes 412.

Scheme 127: Synthesis of bridged 1,2,4,5-tetraoxanes 415a–l from β-diketones 414a–l and H2O2.

Scheme 128: Dimerization of zwitterions 417.

Scheme 129: Ozonolysis of verbenone 419.

Scheme 130: Ozonolysis of O-methyl oxime 424.

Scheme 131: Peroxidation of 1,1,1-trifluorododecan-2-one 426 with oxone.

Scheme 132: Intramolecular cyclization of dialdehyde 428 with H2O2.

Scheme 133: Tetraoxanes 433–435 as by-products in peroxidation of ketals 430–432.

Scheme 134: Transformation of triperoxide 436 in diperoxide 437.

Scheme 135: Preparation and structural modifications of tetraoxanes.

Scheme 136: Structural modifications of steroidal tetraoxanes.

Scheme 137: Synthesis of 1,2,4,5-tetraoxane 454 containing the fluorescent moiety.

Scheme 138: Synthesis of tetraoxane 458 (RKA182).
Synthesis of meso-substituted dihydro-1,3-oxazinoporphyrins
- Satyasheel Sharma and
- Mahendra Nath
Beilstein J. Org. Chem. 2013, 9, 496–502, doi:10.3762/bjoc.9.53
- anticancer significance of these two classes of molecules, it was contemplated to construct new dihydro-1,3-oxazinoporphyrins combining the porphyrin and dihydro-1,3-oxazine moieties in a single molecular framework. Such hybrid compounds may prove useful for pharmacological studies or in the development of

Graphical Abstract

Scheme 1: Synthesis of dihydro-1,3-benzoxazinoporphyrins.

Scheme 2: Synthesis of dihydro-1,3-naphthoxazinoporphyrins.

Scheme 3: Synthesis of naphtho[e]bis(dihydro-1,3-oxazinoporphyrin) derivatives.

Figure 1: (a) Electronic absorption spectra of free-base porphyrins 6, 8, 14, 16 and 18 in CHCl3 at 298 K. (b...






